From Lord Carter’s 2015 report through to Scan4Safety in 2017 mandating GS1 and PEPPOL compliance, the call for improved patient safety through better inventory management prior to and following the COVID-19 pandemic has long been stated. Yet despite these initiatives, many Trusts are still grappling with a lack of the right systems and processes to enable achievement of these aims.
In June 2023 the government published a mandate to address the most urgent needs for the NHS. Amongst the three key priorities outlined, a target was set for all Trusts to adopt barcode scanning of high-risk medical devices and submit data to the national, mandatory Medical Device Outcome Registry, by March 2024.
Nine months later and we’ve seen several programmes and initiatives kick start with the aim of supporting the advancement of patient safety through barcode scanning, including the NHS Supply Chain inventory management programme, which Akeso supported to mobilise, as well as more recently the reinvigoration of the Scan4Safety programme.
Whilst there has been notable investment, a significant number of Trusts do not have the right inventory management systems and processes in place to enable Scan4Safety effectively. Based on our analysis, we understand that almost half of acute Trusts in England do not currently have sufficient capability to meet the mandate requirements set out through barcode scanning capabilities. Furthermore, we estimate that only 30% of acute Trusts have the capability to manage inventory at the point of care and therefore meet Scan4Safety requirements.

Untapped Benefits within Inventory Management
Given the significant gap between Trusts with and without barcode scanning capability, there is an opportunity to tap into the wide-reaching benefits that optimised inventory management can achieve – from improvement to patient safety, greater traceability and operational productivity, to cash-releasing supply chain efficiencies. The below outlines some of the expected benefits Scan4Safety through inventory management optimisation can bring.

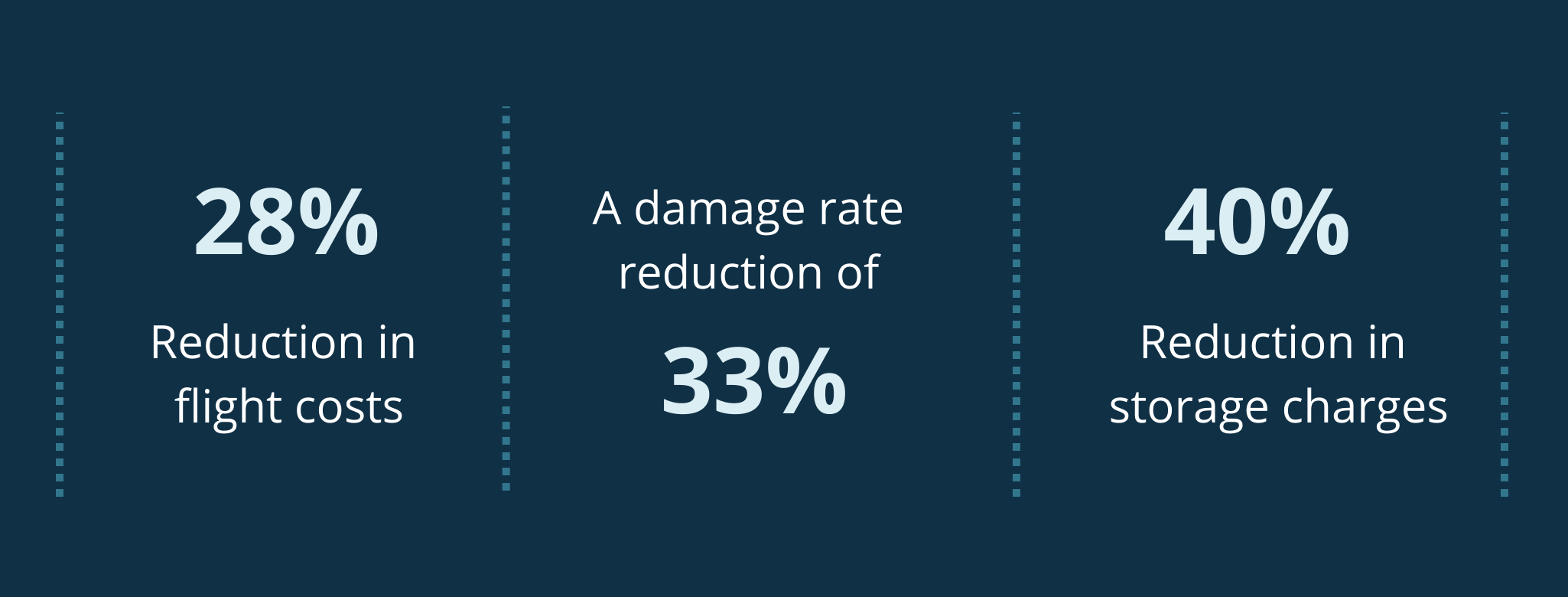
Based on our analysis we estimate that the average Trust could achieve the following key benefits:
- Equivalent of 5 clinical WTE released back to critical patient facing activities
- One-time cash releasing benefit of c. £1 million and recurring financial benefit of £50,000-£100,000
- Wider supply chain and logistics efficiencies through greater visibility and control of ordering as well as improved supplier relationship management
However, despite this, the reality can be quite different for many Trusts. With common barriers, including siloed working across functions and Trust data maturity, understanding the landscape and due consideration to the change required is critical to the success of achieving positive and sustainable change.
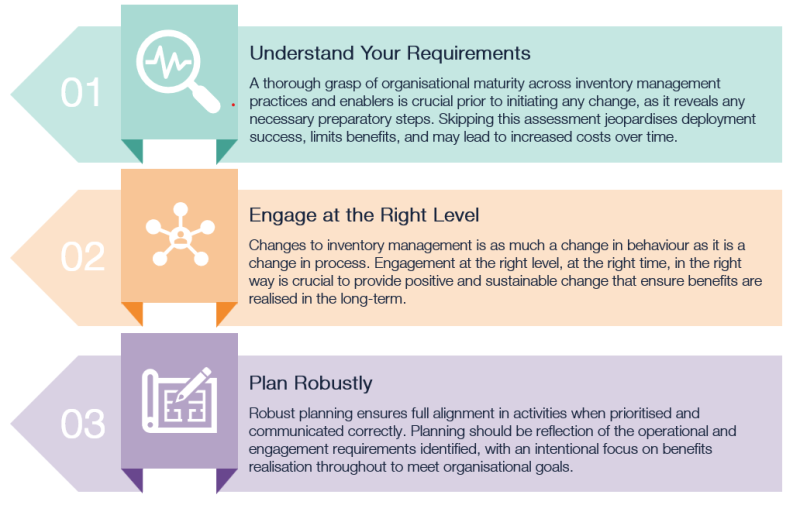
Key Success Criteria
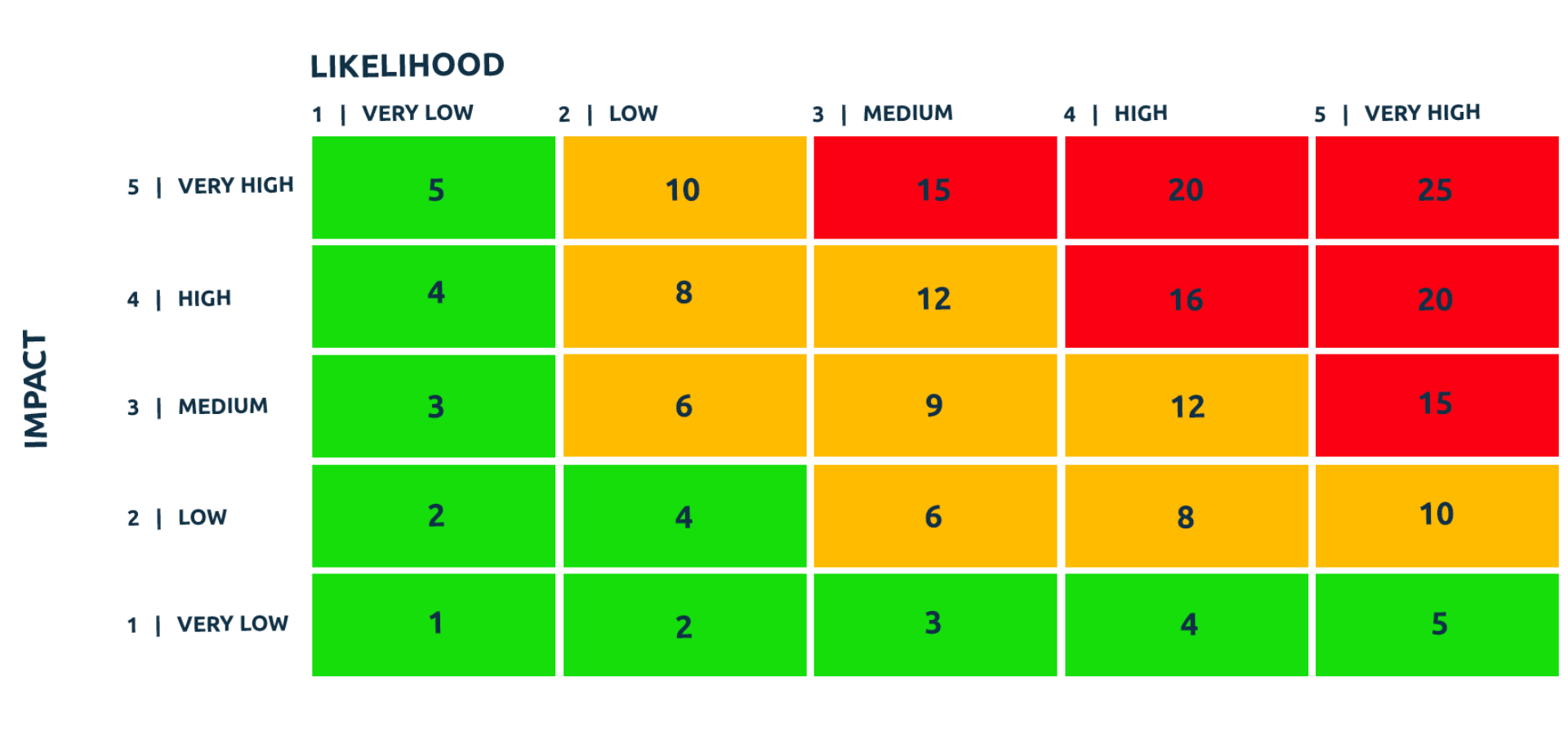
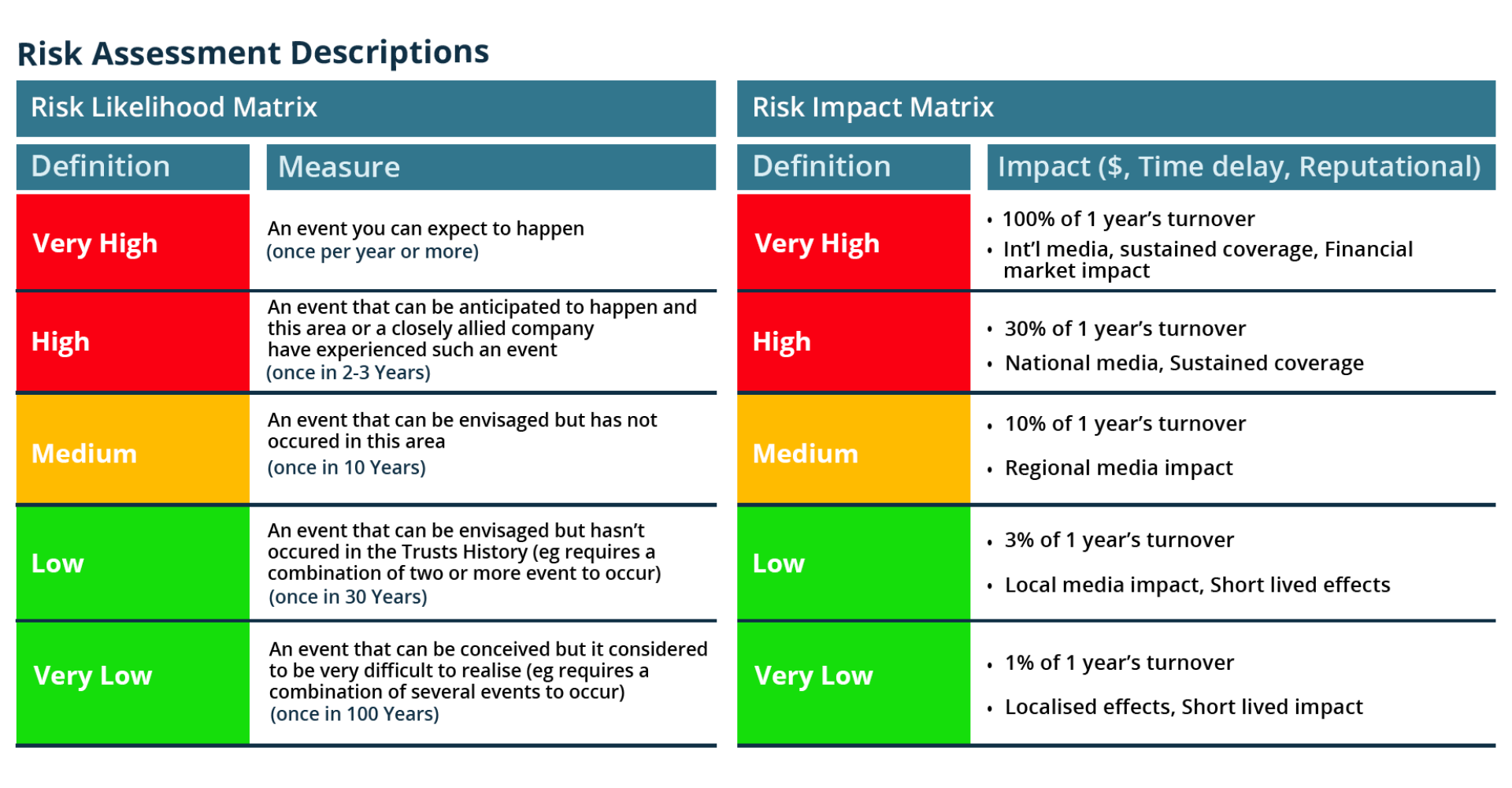
Based on our experience we have summarised the key success criteria that are required to effectively optimise inventory management through barcode scanning. Together these key success criteria make up the core fundamentals which enable inventory management optimisation best practice.
How We Can Support You
Akeso have worked hand-in-hand with a number of Trusts from business case development and benefits modelling through to implementation and benefits realisation, including most recently the establishment of the NHS Supply Chain inventory management programme. As such we are well positioned to support Trusts and ICBs navigate the current landscape and support accelerate your Scan4Safety proposition.
Sign up to access our free ‘how to’ guide for further information on how healthcare organisations can accelerate Scan4Safety through inventory management optimisation:
"*" indicates required fieldsScan4Safety Acceleration Guide