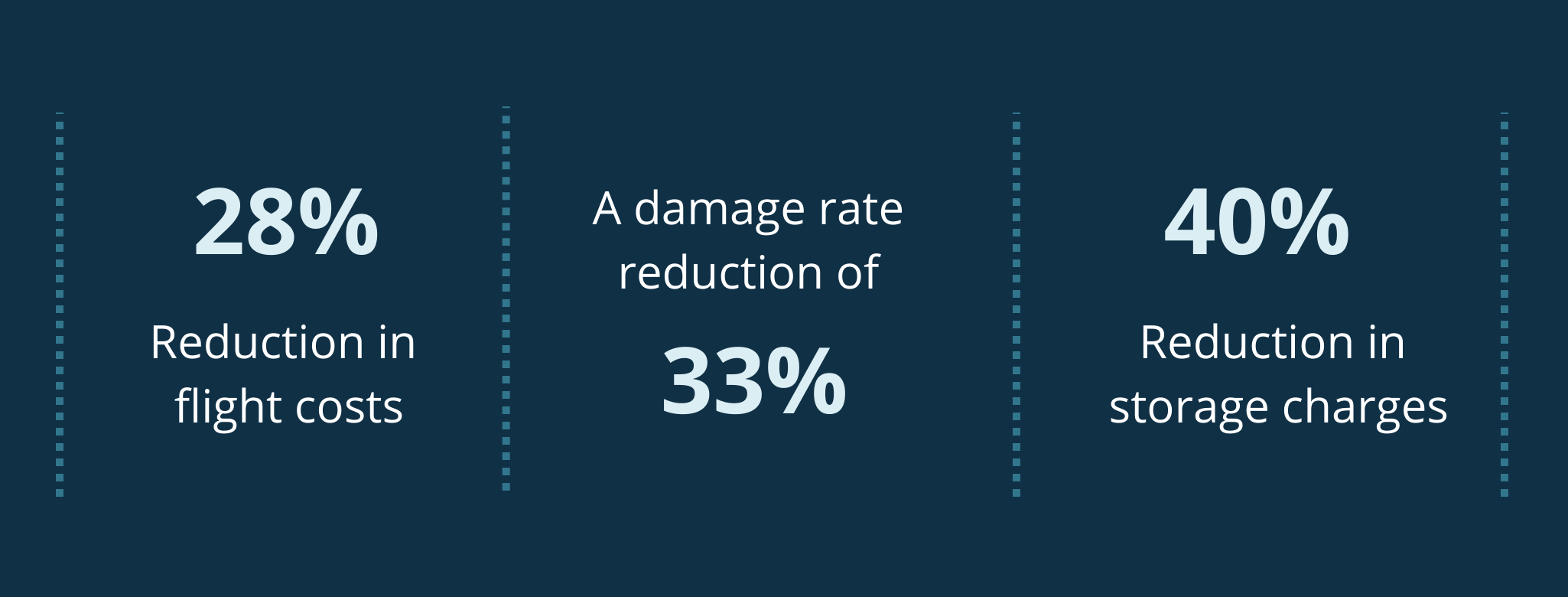
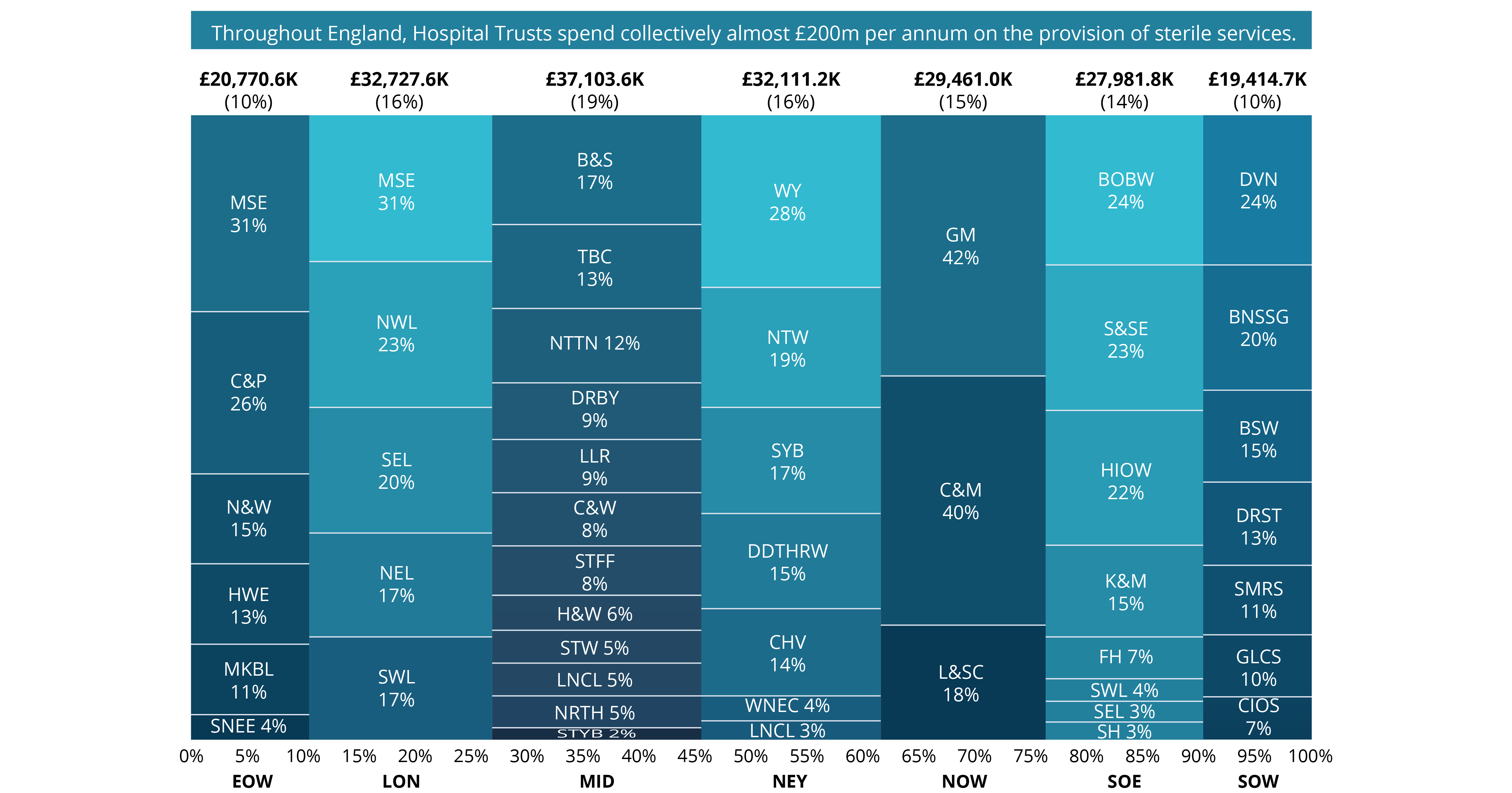
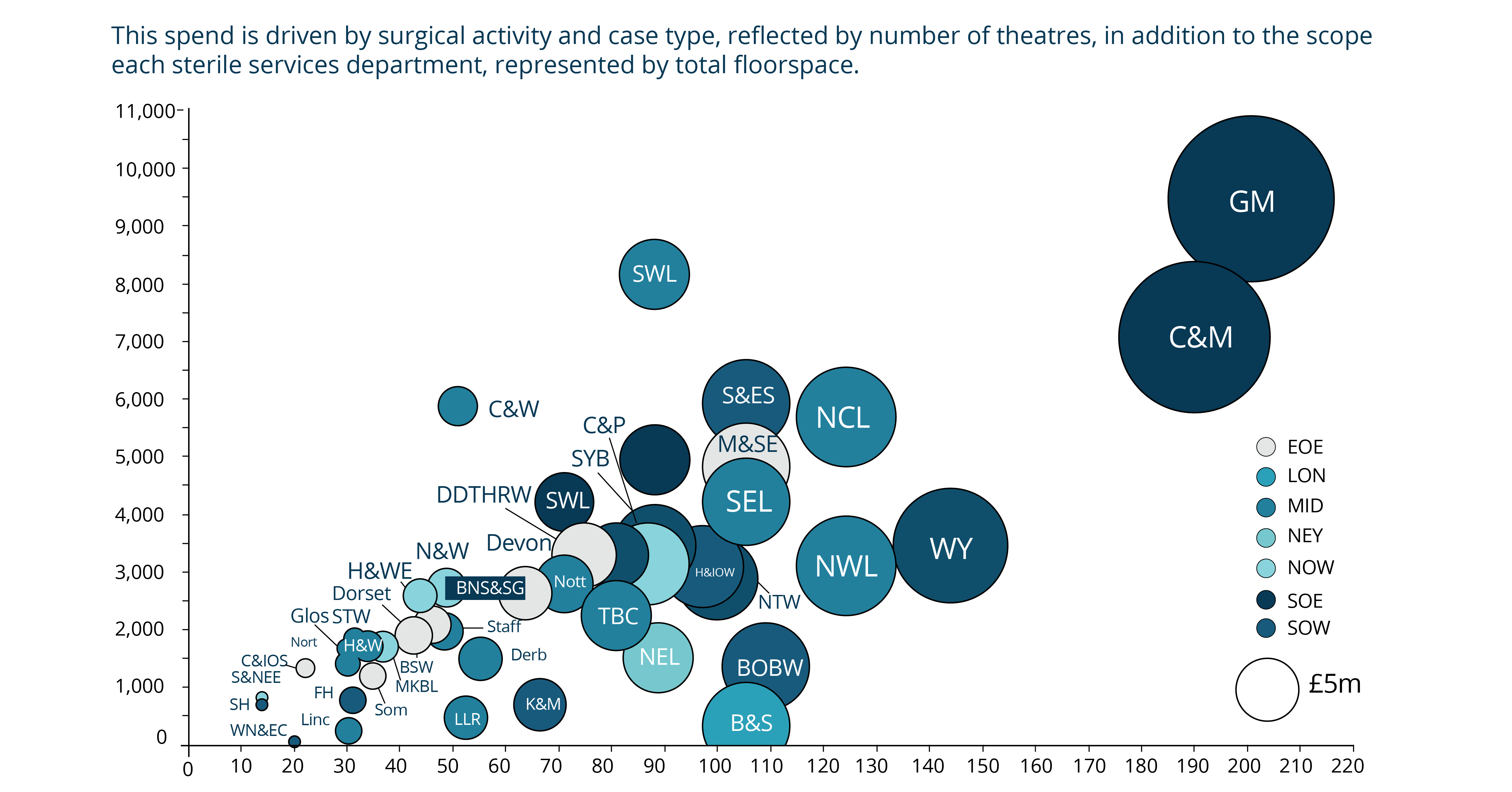
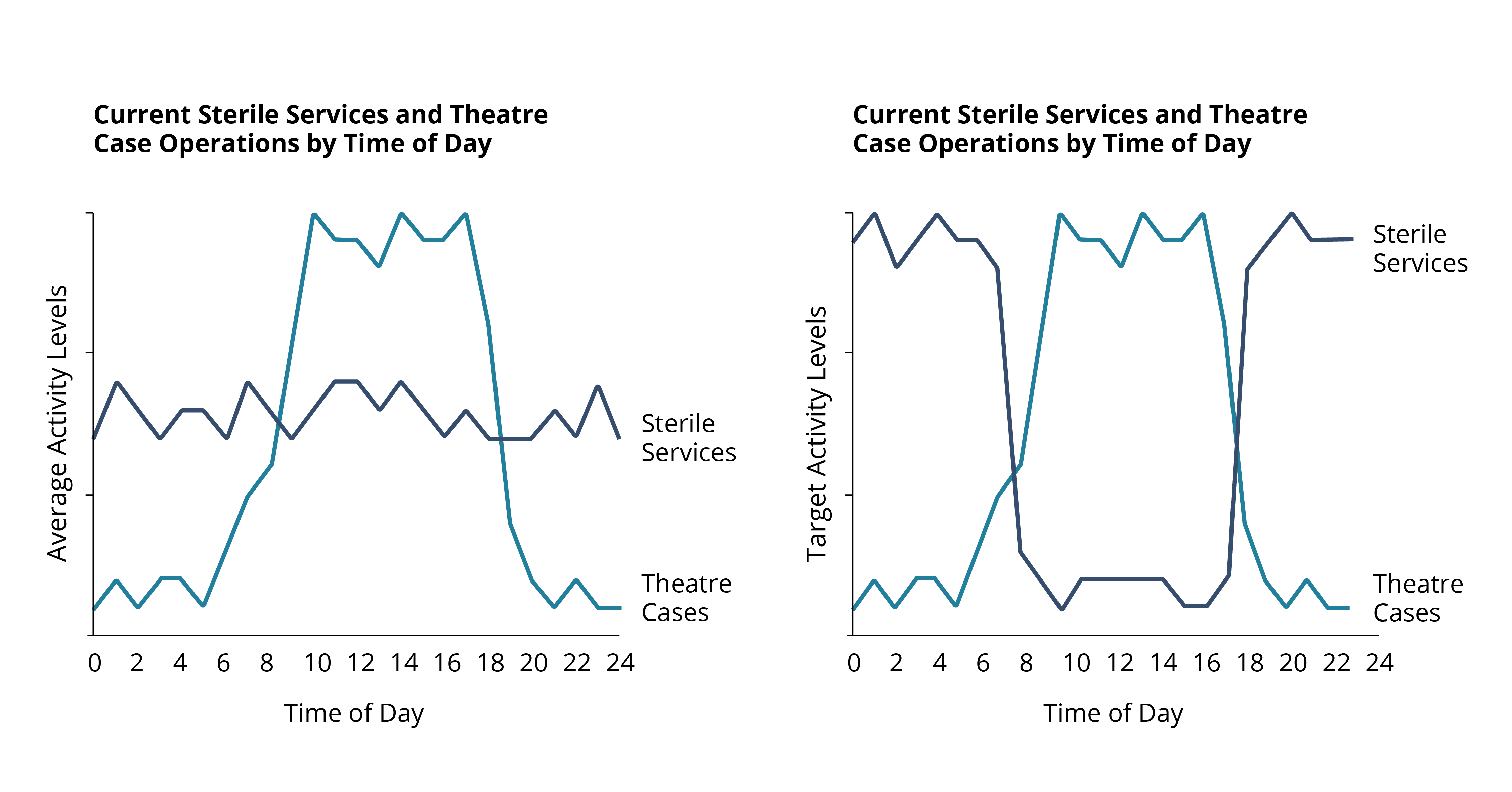
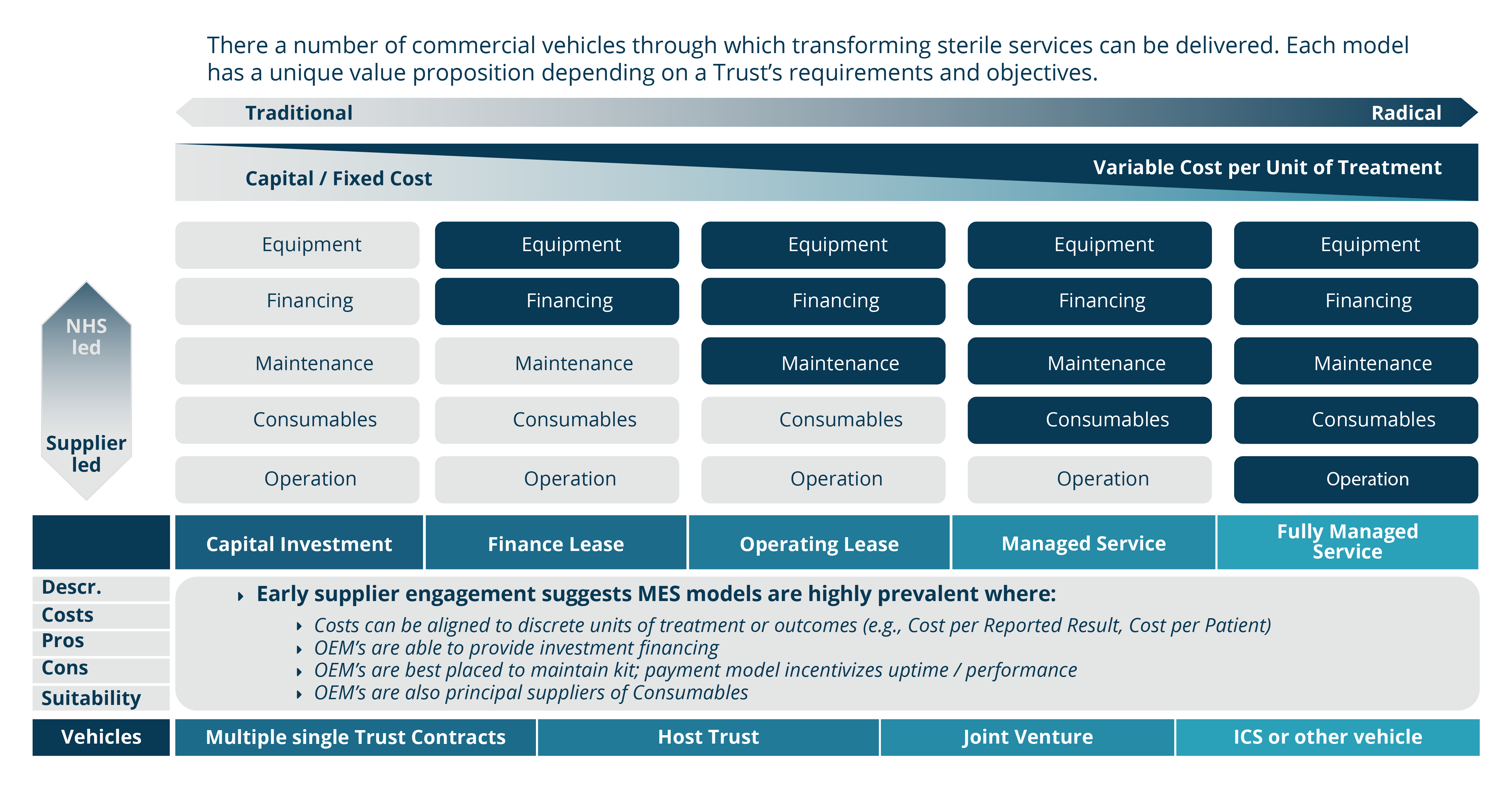
Even before the pandemic began, demand for diagnostic services of all types were rising and, in some cases, outstripping capacity. Covid-19 has exacerbated this problem, deepening the diagnostic backlog with knock-on effects for cancer and elective care.
However, it has also demonstrated what is possible. Seemingly complex changes were implemented at a pace not seen before, transforming services within a matter of weeks to ensure they continued during lockdowns, whilst incorporating Covid-minimisation measures.
Although the challenge of catching up with the diagnostic backlog is a steep one, it presents similar opportunities to deliver much-needed long-term change in diagnostic services.
Reshaping diagnostics for the new normal
The challenges created by Covid-19 are ongoing and require new and sustainable solutions. Standard diagnostic pathways have remained the same for many years, despite the fact they are often inefficient both for the NHS and its patients.
But the drive to develop a system that harnesses new ways of working and new technology has already begun. This includes more than 40 new community diagnostic centres that are currently being rolled out by NHS England and set to provide around 2.8m scans in their first full year of operation. Situated in a range of settings from local shopping centres to football stadiums, they are designed to give patients more direct access to the full range of diagnostic tests closer to home.
Crucial to the success of many of these initiatives, will be the relationship between the community and acute services. The transition to integrated care systems (ICS) will help to create this, but a truly interconnected system will still require wider change.
An independent review of diagnostics services for NHS England recently outlined the key components of a new service delivery model. This article explores some of its recommendations and the barriers Trusts need to overcome to achieve them.
What could the future of community diagnostic services look like?
There are three key models to transforming community diagnostic services.
Optimal care pathways
Building on established pathways through existing community support, such as pharmacists, opticians, and phlebotomy services, is one model of delivery that realises the benefits of a greater separation of acute and elective diagnostics.
This provides patients with quicker and more convenient access to care closer to home or work, whilst relieving pressure on acute sites. Telephone and virtual consultations are also expected to play a much larger role in diagnostic services in the near future.
Optimising these established pathways brings a range of challenges which Trusts need to consider, including:
- Financial arrangements – Ensuring the commercial arrangements are cost-effective and attractive for the commissioner and the service provider, is important in order for services to run smoothly. A comprehensive activity forecast and tested commercial model is critical.
- Care boundaries – Optimising care pathways should involve eliminating the boundaries that still exist in the care system. The transition to an ICS model will play a key role here. As will an effective commercial arrangement that gives the patient true flexibility and choice on where they receive their care in the community.
- Collaborative working – Integrated care requires collaboration on all fronts. Often parity of esteem or an assumption of vested interest builds barriers between professionals. Ensuring clinical professionals are engaged and introduced at an early stage will help alleviate this.
- Public perception – With the introduction of any new service model, comes the requirement for change management. Fear of a patient backlash often deters Trusts from doing this, but regular patient engagement and feedback is vital.
- Social value – An area of increasing priority, the service provider should demonstrate how they will aid recovery of the local community and economy through employment and training, as well as community support.
Community diagnostic hubs
There is an opportunity to develop new diagnostic service models outside the existing Healthcare landscape that are more responsive and innovative, such as community (or remote) diagnostic hubs (CDHs).
They provide a one-stop shop for patients requiring potentially life-saving diagnostic tests closer to home. As noted earlier, centres that deliver this kind of service are already being rolled out and have begun combatting the impacts of Covid-19.
Their numbers and the services they offer are set to grow over the next five years in a bid to reduce the pressure on acute care. In the near term, non-invasive diagnostics are the most viable, but with developments in technology and practice, there will be an increase in more time-consuming, invasive diagnostics in the community.
As part of this initiative, Akeso supported leading specialist Trusts to implement CDHs. There are several key challenges to consider during this process to ensure a successful implementation:
- Strategic vision and scope – Fully define the strategic objectives for the hub before implementation. This will inform the scope and operating model as well as support timely decision making and evaluation.
- Patient need – Identify who the hub’s patients will be and what their needs are. Every aspect of the service model and patient pathway must be built around this.
- Project management office (PMO) – Robust planning from the outset is critical to the success of the project implementation. With involvement from multiple stakeholders, capturing dependencies at each stage will not only ensure the Project is delivered on budget, but also prevent surprises further down the line.
- Resourcing – Identify and engage with the right people early on. Collaborating with clinical and operational people across the organisation, who have the right expertise and experience to implement a new service model will avoid potential setbacks.
- Capacity modelling – Model patient activity across the whole patient pathway. Capacity within the CDH must align with the Trust’s internal capacity. This may be dependent on the capacity to book patients’ assessments and follow-up consultations.
- Service resilience – In light of Covid-19 ensure the safety of patients and service resilience by reviewing patient flow and infection control.
New diagnostic technologies
Innovation is advancing rapidly in areas such as genomic testing, point-of-care testing and the use of artificial intelligence for imaging, endoscopy, and wearable devices. These have the potential to transform the service diagnostic hubs can offer.
Historically Healthcare providers have been slow to adopt new technological innovations. That is why it is important to explore the most effective way to introduce them. Here are some considerations to bear in mind:
- Clinician uptake – Clinicians need to be encouraged to trust the integrity of new technology and move away from established processes.
- Patient awareness – Patients must be supported to understand and adopt new technology. Striking the right balance between a face-to-face and digital service is vital.
- System interoperability – New systems and equipment will need to exchange information seamlessly. Clinical data comes in a variety of formats and terminology, which means standardised catalogues will need to be developed for complete interoperability.
- Safety – Safeguards must be put in place to ensure data compliance, and Healthcare workers are given the time and knowledge to implement them.
Combining these elements will create community diagnostics services that can rise to the challenges created by the pandemic, while also improving patient care.
By considering the key factors mentioned here at the outset, Trusts will be able to successfully implement and operate each element successfully.
As experts in delivering high quality solutions to the Healthcare sector, Akeso has a track record of supporting Trusts to do this in a way that develops the effective diagnostic services of tomorrow. To find out what we offer, get in touch at enquiries@akeso.co.uk